Would you say quality is important for medical devices? Of course you say “Yes!”. Is that also true for custom-made medical devices? Why not? And what about 3D printed custom-made medical devices? Ditto! After all, it’s a medical device and it will be implanted into a patient. For the rest of his/her life. So you would expect the best-of-the-best, super high level quality, no? Yes, but it is not so black and white. Obviously, there is a “risk-benefit” ratio that guides the development of all medical devices; what risk is acceptable while achieving a significant benefit for the patient? In the context of 3D printed titanium cranial plates; What quality level do we minimally need? Will improving the quality even further reduce the risk and increase the benefit? And from a commercial point of view: How perfect should it be, while still being commercially interesting?
Custom-made devices are a special category of medical devices in the MDR. Because only one implant is made for one particular patient, it’s impossible to perform large series of (mostly destructive) tests on representative samples, let alone perform animal studies or clinical trials for each case. Nevertheless, custom-made devices are expected to adhere to the General Safety and Performance Requirements (GSPR). And the manufacturer is expected to have implemented a quality management system (QMS).
However, often it seems that a custom-made device is a free pass to ‘experiment’ (is that the right word?). Poor designs get approved by surgeons (who have, generally speaking, no design expertise) and are then poorly manufactured. And because it’s a one-off, 3D printing is often the go-to technology.
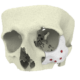
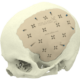
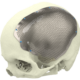
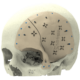
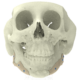
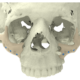
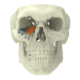
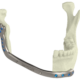
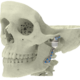
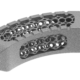
Below you see two examples of titanium cranial plates. The implant on the left is 3D printed by an unknow supplier (let’s call it implant Y) and delivered to a hospital. The cranial implant on the right is a typical Xilloc cranial implant. Implant Y shows a very uneven surface quality, with the top part grinded (probably in removing the support material) and the rest of the surface left ‘as built’. It shows variations in color and scratches. Will implant Y perform less when used for cranial reconstruction? Probably not if the flaws are only cosmetic. Probably yes if the rough surface damages/irritates the overlying tissues and if the color variations are the result of poor heat treatment resulting in a deformed or warped implant. Also the screwholes along the perimeter seem rather large for the tiny fixation screws that are normally used.
Obviously, quality is more than just visual appearance. You can’t judge a book by its cover. Having a quality management system in place cannot be judged by visual appearance of the released products, although you can deduce quite a bit I would say. If you are serious about quality management, you want your parts to look good too, no? Because when the appearance of an implant is bad, I can’t help but wonder about the quality ‘below the surface’: Does it even fit? Is heat treatment for stress relieve performed properly? Is it clean? How low is the bioburden? Are the mechanical properties okay?And even further down the line: How about traceability? What is their powder management? Etc, etc.
Basically, you want to know if the supplier is ISO 13485 certified and under what scope. Because having such a certification means there is a proper quality management system in place ànd the supplier adheres to the applicable regulations (a.o. the MDR). Audits and certification are performed by an independent 3rd party (Certification Body, not to be confused with a Notified Body) and their medical devices adhere to the GSPR. That way, you should be able to trust that the company providing the custom-made implant did it’s homework.
Surprisingly, the MDR only requires manufacturers to have a “quality management system” in place. It’s not required to be certified by a 3rd party. Nor does it need to be ISO 13485. Whereas for manufacturers of standard devices, having an ISO 13485 certified QMS is obvious, it’s apparently not so obvious for manufacturers of custom-made devices. I would strongly recommend it however. Getting such a certification is not an easy task, but I am glad we did it (and we keep doing it, since ‘continuous improvement’ is also part of a QMS and audits happen on a regular basis).
Over the years, not one surgeon has ever asked me if our company is ISO 13485 certified. Maybe they are not aware, but probably because they simply trust their suppliers to deliver ‘quality’. For the sake of the patient, ask your supplier of custom-made implants if they are ISO 13485 certified.
Yet maybe the concept of “quality” is not tangible enough. So, I propose that whenever you see a custom-made implant, ask yourself: “Would I implant it into my child?” If the answer is No, well, then don’t. And if you can’t answer that question immediately, ask an expert.
Erik Boelen, MSc, PhD
Chief Operations Officer | Xilloc Medical