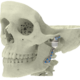
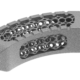
High quality products for an improved quality of life
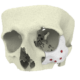
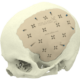
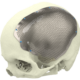
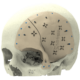
At Xilloc we take pride in providing the highest quality patient-specific devices on the market. To assure the highest quality and to comply with the Medical Device Directive 93/42/EEC (MDD) and the new Medical Device Regulation 2017 (MDR) we implemented a Quality Management System that is evaluated, approved and certified according to EN ISO 13485.

As you can expect from Xilloc as a young and innovative company, we moved from a paper-based to a digital Quality Management System.